Steffanie Strathdee hunched over her laptop, fretting. She barely noticed the kittens asleep next to her or the serene Buddha figure across the living room, anchored next to the glass doors that looked toward the gleaming Pacific. Her mind was 20 miles away in the intensive care unit of the University of California-San Diego’s medical center, where her husband, Tom Patterson, lay in a coma.
Patterson was 68; Strathdee was 49. They had been married 11 years, after meeting in a grant review group convened by the National Institutes of Health. He was a psychologist and she was an infectious-disease epidemiologist; when they fell in love, they also formed a powerhouse research team, studying the effect of the AIDS virus on vulnerable people in Tijuana, Mexico.
But it was a bacterium, not a virus, that was bedeviling them now. Three months earlier, on the last night of a Thanksgiving vacation in Egypt, Patterson had suddenly fallen ill, so severely that he had to be medevaced to Germany and then to UCSD. There were several things wrong—a gallstone, an abscess in his pancreas—but the core of the problem was an infection with a superbug, a bacterium named Acinetobacter baumannii that was resistant to every antibiotic his medical team tried to treat it with. Patterson had been a burly man, 6-foot-5 and more than 300 pounds, but now he was wasted, his cheekbones jutting through his skin. Intravenous lines snaked into his arms and neck, and tubes to carry away seepage pierced his abdomen. He was delirious and his blood pressure was falling, and the medical staff had sedated him and intubated him to make sure he got the oxygen he needed. He was dying.
Strathdee’s friends knew she was desperately searching for solutions, and one told her about an acquaintance with an intractable infection who had traveled to Eastern Europe to seek out a century-old cure. Strathdee spent days reading whatever she could find about it, and now she was composing a last-ditch email to the hospital’s head of infectious diseases, the person who would rule on whether they could use it to help her spouse.
“We are running out of options to save Tom,” she wrote. “What do you think about phage therapy?”
Strathdee didn’t realize it at the time, but her attempt to save her husband’s life would test the bounds of the American medical system—and throw its limitations into stark relief.
Listen to Tom Patterson and Steffanie Strathdee recount their desperate search for answers and the history of phage therapy, on this episode of the Mother Jones Podcast:
The treatment Strathdee had fixed on as a last-ditch hope is almost never used in the United States. The Food and Drug Administration has not licensed phage therapy, keeping it out of pharmacies and hospitals. Few physicians have used it even experimentally, and most civilians have never heard of it. But phages are a natural phenomenon, frequently deployed in the former Soviet Union. When used properly, they can save lives.
To understand how phage therapy works, it helps to know a little biology, starting with the distinction between bacteria and viruses. Most of the drug-resistant superbugs that cause medical havoc are bacteria, microscopic single-celled organisms that do most of the things that other living things do: seek nutrition, metabolize it into energy, produce offspring. Viruses, which are much smaller than bacteria, exist only to reproduce: They attach to a cell, hijack its reproductive machinery to make fresh viruses, and then, in most cases, explode the cell to let viral copies float free.
Phages are viruses. In the wild, they are the cleanup crew that keeps bacteria from taking over the world. Bacteria reproduce relentlessly, a new generation every 20 minutes or so, and phages kill them just as rapidly, preventing the burgeoning bacterial biomass from swamping the planet like a B-movie slime monster. But phages do not kill indiscriminately: Though there are trillions in the world, each is tuned evolutionarily to destroy only particular bacteria. In 1917, a self-taught microbiologist named Félix d’Herelle recognized phages’ talent for targeted killing. He imagined that if he could find the correct phages, he could use them to cure deadly bacterial infections.
That was a gleaming hope, because at the time, nothing else could. (Sir Alexander Fleming wouldn’t find the mold that makes penicillin, the first antibiotic, until 1928.) Treatments were primitive: aspirin and ice baths to knock down fever, injections of crude immunotherapy extracted from the blood of horses and sheep, and amputation when a scratch or cut let infection burgeon in a limb and threaten the rest of the body with sepsis. Phages—whose full name, bacteriophages (or “bacteria eaters”), was given by d’Herelle in 1916—did something that medicine had never before been able to accomplish: They vanquished the infections for which they were administered without otherwise harming patients. A medical sensation and a cultural phenomenon, they provided the key plot device in the novel Arrowsmith, about an idealistic doctor, that won the Pulitzer Prize in 1926, and they saved the life of the Hollywood cowboy actor Tom Mix, a 1930s superstar.
D’Herelle was a restless researcher who seems to have felt undervalued despite being awarded jobs in Paris and Vietnam and at Yale. That insecurity made him vulnerable to an offer he received in 1933 to relocate to Tbilisi in Georgia, home territory of Soviet dictator Joseph Stalin. With a protégé, Georgi Eliava, d’Herelle co-founded the Eliava Institute of Bacteriophages, Microbiology and Virology. Stalin showered the institute with attention and money because it offered something he badly wanted: a scientific achievement that he could portray as a pure product of communism. Antibiotics became the basis of infectious-disease medicine in the West, but behind the Iron Curtain, phages took their place.
Eliava was murdered in a political purge in 1937, and d’Herelle died in 1949. Their institute dwindled, but it survived the collapse of the Soviet Union in 1991 and the Georgian civil war the following year. When the former USSR opened up to the West, physicians in the United States and Europe learned the Eliava Institute was one of the few places in the world where researchers were still studying and administering phages. That was fortunate timing, because antibiotics in the West were losing their power under the onslaught of antibiotic resistance.
Antibiotics began as natural compounds, the chemical weapons that bacteria aim against each other to compete for living space and food. For millennia before humans arrived, bacteria countered those attacks with mutations—and when humans turned those natural weapons into medicine, by taking them into laboratories to synthesize and perfect them, bacteria kept on adapting. The mutations they produced in response to antibiotics are what we call antibiotic resistance.

How Factory Farms Play Chicken With Antibiotics
Penicillin-resistant staph infections swept the world not long after penicillin came into use during World War II. Methicillin-resistant staph (MRSA) immediately followed the 1960 debut of methicillin, designed to replace some of penicillin’s lost firepower. Over the decades, as each new antibiotic arrived, resistant infections have arisen to undermine them. In the United States, the Centers for Disease Control and Prevention estimated in 2013 that at least 23,000 people die each year from resistant infections, and that 2 million are made sick enough to go to a doctor’s office or hospital. Worldwide, the death toll is estimated at 700,000 people a year. And because resistance is accelerating ahead of production of new drugs to counter it, the death toll is expected to rise to 10 million per year and cost the world as much as $100 trillion in lost economic activity by 2050.
Superbugs pervade health care, causing grave infections after surgeries and in intensive care units, and because antibiotics are routinely added to livestock feed, they permeate the food supply. In 2015, for instance, an FDA project discovered that 47 percent of salmonella bacteria samples found in retail chicken were resistant to tetracycline, as were 76 percent of the E. coli found in ground turkey.
Phages’ vast biological diversity helps them against the mutations that make up disease organisms’ resistance defenses. Plus, because phages kill only specific strains of bacteria, they can quell infections without inducing a terrible diarrheal disease from Clostridium difficile (usually known as C. diff) that occurs when the balance of bacteria in the gut is disrupted by antibiotics wiping out good bugs along with the bad. The CDC estimates that in 2011 there were more than 450,000 cases of C. diff infections in the United States, leading to more than 15,000 deaths. It’s possible that using phage therapy instead of antibiotics could prevent some of them.
But for phage therapy to be deployed routinely in the United States, phages would have to be approved as drugs by the FDA. To treat an American patient with them now requires emergency compassionate-use authorization—effectively an acknowledgment that nothing with an FDA license can save the patient’s life. And Strathdee was about to learn that because phages have no such approval, awareness of them is scarce and unevenly distributed, and finding the right researchers and physicians requires extraordinary luck.
Strathdee directs UCSD’s Global Health Institute and like her husband is a professor in the medical school. Decades earlier, she had tinkered with phages in lab science classes, using them as a tool to differentiate bacteria. Before her husband got sick, she had never heard they could be used as treatments.
The physician whom she’d emailed—Dr. Robert “Chip” Schooley, at the time UCSD’s chief of infectious diseases, and an old friend—knew a little more. Anyone who works in infectious diseases is aware of the peril of drug resistance, and the wish for reliable alternatives to antibiotics is a constant companion to that work. But phages had no direct relevance for him because his personal expertise is disease-causing viruses—HIV and hepatitis—that phages would not affect.
He knew the next step to take, though. The FDA maintains a hotline that lets physicians ask permission to use an unapproved treatment on a single patient if every other hope has been exhausted. The FDA’s reviewer agreed to let the pair attempt phages.

David Walter Banks
Time was short, and the odds were against Strathdee. She needed to find someone who was conducting phage research, who had already isolated phages that worked against Acinetobacter, and who would be willing to test those phages on Patterson’s infection to see if there was a match.
“She has persuasive power,” Young recalled. “And she has made herself probably the most knowledgeable civilian in the world on the use of phage therapy. She got us mobilized.” She cast a wide net, sending about 10 emails to labs around the world, including the Eliava Institute. Two and a half hours later, she got a message from one of the few phage research groups in the United States, the Center for Phage Technology at Texas A&M University, run by a biologist named Ryland Young. Strathdee called Young and talked at him for more than an hour.
What happened next illustrates how time-consuming it can be to try to use an unapproved treatment. Young’s lab has been isolating and testing phages since 2010 and has several hundred individual viruses in its collection, but when Strathdee called fewer than 10 of them were known to work against Acinetobacter. Young put out a call to the small worldwide network of phage researchers, asking for contributions, and was sent some 35 new viruses. He and his lab tested all of them on a sample from Patterson’s infection, sent by Schooley. None of Young’s phages made a dent. One virus, sent by a company called AmpliPhi, did kill cells from the infection. They would need more to make a difference, so Young and his team embarked on what he drawlingly calls “a good old-fashioned phage hunt.” To find a phage that worked against Acinetobacter, Young reasoned, he would have to go look for Acinetobacter in the wild. So he sent his students hunting for environmental samples, dipping into effluent from sewage-treatment plants, pulling water from ponds, and taking swabs of pigs on ranches near the university. Among the 126 samples obtained during the expedition, the Texas lab identified three phages that worked against Patterson’s strain of Acinetobacter.
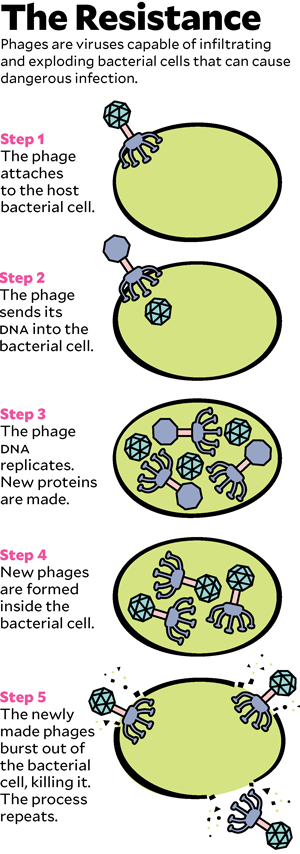
Now they had individual viruses that might do the trick—but they needed to grow enough of them to make up a treatment. They let the bacteria from Patterson’s infection reproduce under lab conditions and then unleashed the phages on them. The viruses worked the way they had evolved to: They attached to the bacteria, inserted their DNA, copied themselves, and exploded the pathogens. The team fed the phages more and more Acinetobacter. In 10 days, they had trillions of copies. Young shipped them in a refrigerated box to Schooley, who meanwhile had been explaining to the university’s biohazard-safety committee why letting a minimally tested living virus into an ICU full of very sick people would not be a risk. (If the phage escaped, it would affect only patients who happened to have the exact same infection as Patterson, and no one else there did.) Schooley had also found another source of phages, in a lab maintained by the US Navy. In tests, four of the Navy phages killed the bacterium from Patterson’s infection as well.
The Texas phages arrived in San Diego first, all four of them combined into a cocktail to increase the odds of success. They had to be scrubbed of cellular toxins and debris from the bacteria they had been grown on, because those contaminants could have sent Patterson into shock. Schooley and his team infused the clean solution into the drains that pierced Patterson’s abdomen, hoping to sterilize the cavities where the infection was lurking. That was on a Tuesday. Patterson didn’t get better, but he also didn’t get worse—encouraging, given how rapidly he had been slipping away. Two days later, the Navy phages arrived, and the UCSD team took a gamble and gave them to him intravenously, to chase the bacteria that had found homes in his lungs and bladder and blood. That was on a Thursday. On Saturday night, Patterson awoke from his coma and recognized his daughter. The phages had done their work.
He was not yet cured, not by a long shot. His infection surged and he crashed back into septic shock the next week, only to be brought out of it with more phages. The same thing happened again a month later, and this time the Navy lab analyzed his infection and tinkered with the phage cocktail.
The whole treatment process was a scramble. “We had two people working literally 24/7 for six weeks to find and supply phages to the clinical team at UCSD,” Young said. “That is not sustainable.”
The effort was such an emergency, Young added, that his group did not have time to fully analyze the phages they sent. Later they discovered that almost all the viruses used in the first round of Patterson’s treatment, both from Texas A&M and from the Navy, targeted the same single attachment point on the outside of the bacterium. It was as if they were all the same drug, instead of eight different ones. That meant the bacterium had to make just one small mutational change to defend itself against them, producing phage resistance—a problem that appears in only a small number of scientific papers about phages and that medicine has not yet had to develop strategies against, because phages have not been a treatment in most of the world.
“If we had been able to do genetic and molecular analysis of the phages, we could have avoided that,” Young said. “The ideal thing would be to have a walk-in cooler of thousands of phages, each of which you know everything about.”
Actually, two decades ago, someone attempted to do just that. Alexander Sulakvelidze, who holds a doctorate in microbiology, is a native of Tbilisi, the home of the Eliava Institute. Sulakvelidze grew up experiencing phage treatments as a routine part of medical care, off-the-shelf products that doctors would prescribe like Western physicians prescribe antibiotics. Then he came to the United States to serve a postdoctoral fellowship at the University of Maryland School of Medicine. One day during his fellowship, his supervisor, Dr. J. Glenn Morris, announced that a patient was gravely ill with a resistant bug called VRE and would likely die.
“I asked him, ‘Why can’t bacteriophages get rid of the VRE?'” Sulakvelidze recalls. “I thought it was a naive question.” But later, after the patient died, Sulakvelidze says he realized, “Something very strange is going on. Somebody just died in the most developed country in the world, from something that could probably be very easily cured in a country like Georgia.”
Out of that realization, Sulakvelidze and Morris and a handful of other researchers formed a company, Intralytix, in 1998. They set out to license phage treatments for VRE, considered at the time the most dangerous of the superbugs. It did not go as planned.
“The investors had no idea what the risks are, the patentability, the return on investment,” he said. “The regulatory agencies had no idea how to regulate this. It was a huge uncertainty.”
This was only a few years after the opening of the USSR. Most of the research written about phages had never been published in English and never evaluated by Western scientists. To make a case for phages in American medicine, clinical trials such as the ones that prove antibiotics’ efficacy would have to be conducted.
But here was the problem. To be approved, an antibiotic must at least reliably kill the common strains and subtypes of the bacteria that cause a particular infection; the broadest-spectrum antibiotics, which doctors usually reach for first, kill multiple species in several groups. But phages do not work against entire groups or even against species. They are weirdly specific and attack bacteria (or not) based on minute genetic differences.
Clinical trials of antibiotics—which progress through three phases before approval and in the third phase can include thousands of patients—are constructed to prove a compound is safe and effective and causes a cure, no matter what minor genetic differences exist from one infection to another. Phages cannot pass that test, because any one phage will only work on a subset of patients.
After hitting a roadblock with the FDA, Sulakvelidze and Intralytix canceled the plan to try to get phages approved as drugs. But they had discovered another opportunity: Food safety is regulated by a different FDA division than drugs are. The company pivoted to isolating phages that would kill the most important foodborne-illness organisms—listeria, salmonella, Shigella, and E. coli. Between 2012 and 2016, the FDA’s food safety arm granted “generally recognized as safe” status—a much lower bar than a new drug approval—to phage cocktails targeting three of the food safety bugs. Sulakvelidze thinks the FDA’s comfort with phages for food is an opening. He is on track to begin trials of a new human product this year.
It’s impossible to know whether Sulakvelidze will be successful, because the FDA says federal law prohibits it from talking about the process of possibly licensing phages for medical purposes. The agency seems to take the position that since it might someday be required to rule on drug licensing for phages, it can’t give any information now about why there have been so few licensing attempts. It won’t even comment on how many licensing attempts there have been, though the ClinicalTrials.gov database maintained by the National Institutes of Health shows that in the United States over the past two decades, 15 studies have used phages: Just two applied phages as a treatment and got through phase one—which uses a small group of people to test safety but doesn’t test efficacy—and those studies did not proceed. The FDA declined to make any of its scientists available for an interview. A spokeswoman, Megan McSeveney, said in a statement that the agency “stands willing to work with bacteriophage developers to provide scientific guidance and clarify regulatory and data requirements necessary to move these products forward in development as quickly as possible.”
The NIH, which focuses purely on research and isn’t responsible for drug licensure, was a little more forthcoming. “Given the problems that we have with antibiotic resistance in this country and throughout the world, it certainly behooves us to explore alternative means of controlling and fighting and countering bacterial infections,” Dr. Anthony Fauci, director of the NIH’s National Institute of Allergy and Infectious Diseases, told me. “Certainly phage therapy is one of those.” In 2016, Fauci said, the NIH wrote about $5 million in grants to medical research centers to gather data on antibiotic alternatives, including phage therapy’s potential against “a variety of recalcitrant infections.” (The US medical establishment isn’t alone in struggling with phage research; the first major EU-backed clinical trial, called Phagoburn, did not proceed beyond phase two.)
Elsewhere in the NIH, Randall Kincaid, a pharmacologist and the senior scientific officer in what’s called the concept acceleration program, explained that launching new categories of treatments isn’t as simple as working out the right structure for a clinical trial. The research has to be worth the end result, he said—which means knowing that physicians will use the treatments when the compounds enter the market, and also that pharmacy managers will buy them.
“You have spectacular life-and-death stories, and everyone wishes to see this pushed forward as concerns about antimicrobial resistance increase, yet you have the real dilemma of demonstrating that these are in fact reliable,” he said. And commercially viable: Since phages are hyperspecific and can’t be pulled off a pharmacy shelf as an antibiotic can, physicians may be deterred from seeking them out, he added—and that would make the trouble and expense of clinical trials pointless.
A set of treatments that the FDA recently accepted might show a path forward for testing and approving phages. Personalized cancer treatments known as CAR-T (for “chimeric antigen receptor T-cell therapy”) involve extracting immune system cells from a patient’s blood so they can be genetically modified in a lab and then reinfused into the patient. In 2017, the FDA gave licenses to two CAR-T treatments, Kymriah for advanced leukemia and Yescarta for a type of lymphoma.
Like phages, CAR-T treatments are tuned to an individual patient. But here’s a key difference that made drug developers think CAR-T was worth pursuing for two decades: If it goes into widespread use, it will make manufacturers tons of money. The cost of a single dose of Kymriah is projected to be a breathtaking $475,000. But antibiotics have never been priced anywhere near as high as cancer drugs, and it seems unlikely that prices would rise for the phages that might supplant them. Those low prices have historically been one reason it has been hard to get drug companies to develop new antibiotics. Consider: In contrast to Kymriah, the antibiotic Avycaz, hailed as a major advance when it was approved in 2015 for hospitalized cases of grave drug-resistant pneumonia, costs as little as $3,500 on price lists and rarely rises above $15,000—and that’s for 10 doses.
For phages to justify investment, developers will have to make a case for using them not just to save the 23,000 people who die of resistant infections in the United States each year, but also to treat some of the 2 million who seek a doctor’s help or hospital care. There are applications: Phages could coat artificial joints and heart valves to prevent pathogenic bacteria from developing sticky drug-resistant mats called biofilms. Phage solutions could be infused into the raw surfaces of diabetic foot ulcers, which are hard to treat because they have a thick, fibrous backing that prevents intravenous drugs from penetrating; that is partly why diabetic patients were among the first victims of VRSA, a staph resistant to even the last-resort antibiotic vancomycin. Intralytix has proposed using phages to kill disease bacteria that are picked up from food and water and live quiescently in the gut for unpredictable periods of time—the superbugs NDM and MCR, which originated in India and China, spread around the world that way. The process of clearing out bad bacteria that aren’t currently causing an infection is called decolonization, and it’s difficult to accomplish with antibiotics, which kill good cells along with bad ones. Phages could be more targeted.
A handful of companies still believe in the possibility of phages, enough to invest in research while the FDA works out its issues. One is AmpliPhi Biosciences, which conducted one of those phase-one treatment trials in the NIH database. AmpliPhi sits north of the UCSD medical center where Patterson was treated, and its phages were among those that became part of his treatment after Young at Texas A&M launched his worldwide plea for phages that might help.
Paul Grint, AmpliPhi’s CEO, is a physician who was a successful antibiotic developer earlier in his career and understands from the inside the Catch-22 of the federal research bureaucracy. The company’s phase-one trial investigated the safety (but not the effectiveness) of a cocktail of three phages that work against drug-resistant staph bacteria by applying the solution to the forearm skin of healthy volunteers. With that box checked, AmpliPhi has been strategizing how to move to the next step, which requires using the cocktail in patients who are experiencing staph infections.
Grint’s solution is to patiently assemble an array of single cases, by contributing his company’s phages to cases such as Patterson’s. “We’ve set a goal of, say, 10 by the end of this year, and 10 to 15 in the early part of next year,” he told me in 2017. “We will then have a data set that allows us to better design a phase-two study and answer some of the questions that regulators have.”The FDA’s emergency exemptions only allow for treating one patient at a time. But in research, one case is an anecdote. To demonstrate to the FDA that a phase-two trial would be safe, the developers need more data than a single case can give them.
But while companies and the FDA negotiate, patients need saving now. In October last year, a 25-year-old woman in Pittsburgh named Mallory Smith, who had cystic fibrosis and had received a lung transplant, developed an infection in her new lungs with a stubborn bacterium named Burkholderia cepacia, to which CF patients are more susceptible. The infection was resistant to every antibiotic her physicians treated it with. Her father, who had heard of Patterson’s ordeal, turned to Strathdee for help.
On November 7, Strathdee posted a plea on Twitter, asking scientists for any phages that might have a hope of matching: “#Phage researchers! I am working with a team to get Burkholderia cepacia phages to treat a 25 y old woman with CF whose infection has failed all #antibiotics. We need…phage URGENTLY to find suitable phage matches.” Of the several hundred phages from around the world that Strathdee was offered, Smith’s father recalls that at the last minute two looked like a match. They were rushed to Smith’s hospital and administered, but it was too late. Mallory Smith died November 15.
Patterson, however, made it. He left the hospital in mid-August 2016—gaunt and weak, having lost most of his muscle mass but having beaten the superbug using phages. He was the first person in the United States to have been successfully treated intravenously.

David Walter Banks
He is still frail; the last-resort antibiotics he was given before the phage treatment temporarily harmed his kidneys. On the day I met him in their home in Carlsbad, California, he had just taken a nap, and he talked to me from a recliner, with a blanket and a cat stretched across his lap.
“I’ve studied AIDS for many, many years, since the beginning of the epidemic, and I always thought viruses were the bad guys, evil,” he said. “Now that I’ve gone through what I have, I can see that viruses may actually be used for good, too.”
Strathdee, who is working on a book with Patterson about their experience, says she hopes to see phages become a routine option for serious infections, available to substitute for antibiotics or to be administered alongside them, given early in treatment and not as a desperate last resort when nothing else may work well.
“It certainly seems to me a lot less risky than antibiotics,” she said. “They’re self-limiting: When the bacteria they attack are gone, they’re gone. That’s a pretty good designer drug, and nature gave it to us.”